


The miss rates of corona antigen self-tests as reported in practice are quite shocking compared those reported in the self-test labelling. What causes those misses? What do we know based on data from clinical practice?
I looked at 6 reasons (and a bonus reason) that may explain why self-tests miss a diagnosis, and checked the reasons against available data from the RIVM and Foundation for Innovative New Diagnostics (FIND):
Today, reason 1 and 2 that explain why the miss rates are so high.
Essentially, self-tests are the same antigen rapid tests as professionals use, but then individually packaged. Plus they have been through some extra administrative procedures (so NO clinical tests or user testing) – after which the government gave them an exemption for use as a self-test.
Antigen tests for professional use are CE marked under the IVD directive (In Vitro Diagnostics Directive 98/79/EC), which is in its last year and will be replaced by the IVDR on May 26, 2022 (IVD regulation, a European law). Manufacturers have bench-marked them against PCR tests to obtain numbers on sensitivity and specificity, and have SELF-certified them for CE mark – something which will no longer be permitted under IVDR, when test for life-threatening diseases such as Covid become class D a.k.a. the “high-risk devices”-class.
Rapid tests have a high miss rate compared to PCR tests, as the worst case theoretical miss rate of self-tests is 1 out of 3 (page 12).
The instructions for use in the packages of self-tests report sensitivities from 83,3% to 97,1%, or in other words, miss rates between 16,7% and 2,9%. The current state-of-the-art expectation for corona tests is a specificity of at least 90% or a miss rate of less than 10%. Theoretical values of self-tests mostly fall within this range, though the worst case values are below 90% for about half of the self-tests.
Taking into account the lower boundary of the sensitivity the lowest value reported is as low as 67%, or a miss rate of 1 out of 3.
That makes the self-tests less reliable than a PCR test.
Though the individual results of self-tests differ, the picture is consistent: all tests validated in professional use settings have a higher miss rate in practice than in the IFU. Miss rates lie between 1 out of 3 to 1 out of 4.
Three of the self-tests for sale have been validated when used by professionals. See the picture for the difference between the miss rate in the IFU (on the left) and the one in clinical practice (on the right, RIVM data, average calculated by me).
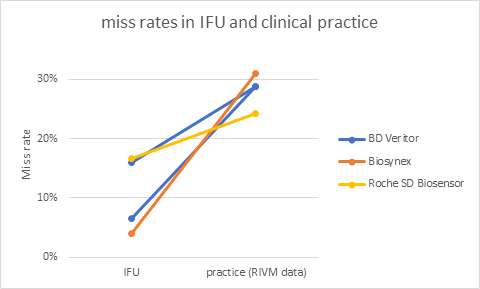
Roche SD Biosensor test is the test that is most widely validated, and sensitivity varies widely: worst case, professionals in an emergency care setting miss almost 1 out of 2 (3 tests, 54 PCR+, table 5) and best case they miss only 1 out of 20 (5,6% ; 1 test, 18 PCR+).
Professionals using these tests miss on average 1 out of 3 to one out of 4 corona cases. What would happen if you gave these tests to you and me, who have no experience in nose swabbing and swab wringing? Can you imagine?
So in short, antigen self-tests may miss a quite a few corona cases because
In my next posts, I will give more reasons why self-tests have quite shocking miss rates. If you can’t wait, please PM or call me ( +31 26 800 1219).
Do you need help getting the right clinical data to obtain a CE mark for a SARS-COV2 (self) test under IVDR (upclassified from self-certification under IVD to the highest risk class, class D under IVDR)? Do you need with setting up a performance evaluation and/or clinical tests for any other upclassified IVD? Do you need help determining if your IVD may be part of the 80% IVDs that are upclassified? Please do not hesitate to reach out! (+31 26 800 1219).
Ines,
Enschede, 18 July, 2021