MDCG issues guidance on content and synopsis of the Clinical Investigation Plan for Medical Device studies |
The MDCG issued guidance regarding the content and the synopsis of the Clinical Investigation Plan (CIP) for Medical Device studies.
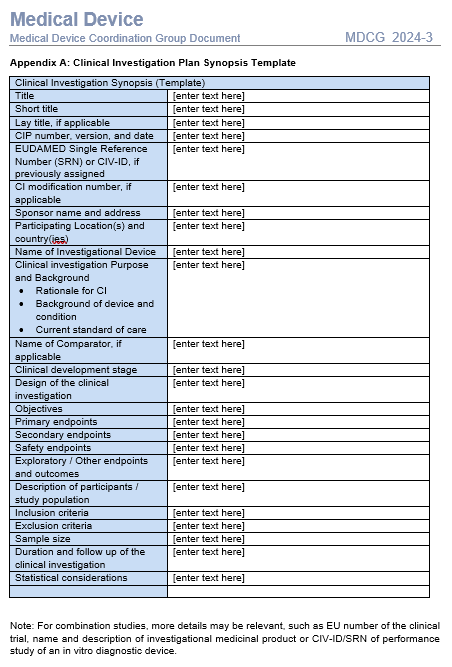
A quick review learns there are no big surprise here:
- The template for the synopsis (MDCG 2024-3 Appendix A) though, does ask for the Clinical development stage (typically only described in the CIP itself) and the EUDAMED number, but not for the study assessments throughout the study (giving an idea regarding the burden for the subjects.
- The guidance regarding the content of the CIP does not reveal big surprises either when you are used to following the guidance from ISO14155 and local Regulatory Bodies, although there is a lot of emphasis regarding some topics like
- the benefits and risks and it’s ratio related to participation in the trial,
- the presence of an implant card in case of a trial with implantables
- listings of foreseeable AE’s and anticipated ADE’s
- statements that need to be included: regarding compliance with good clinical practices and the MDR, and regarding publication and authorship,
- a description of the arrangements for care of trial participants after their participation has ended
-
to name a few.


