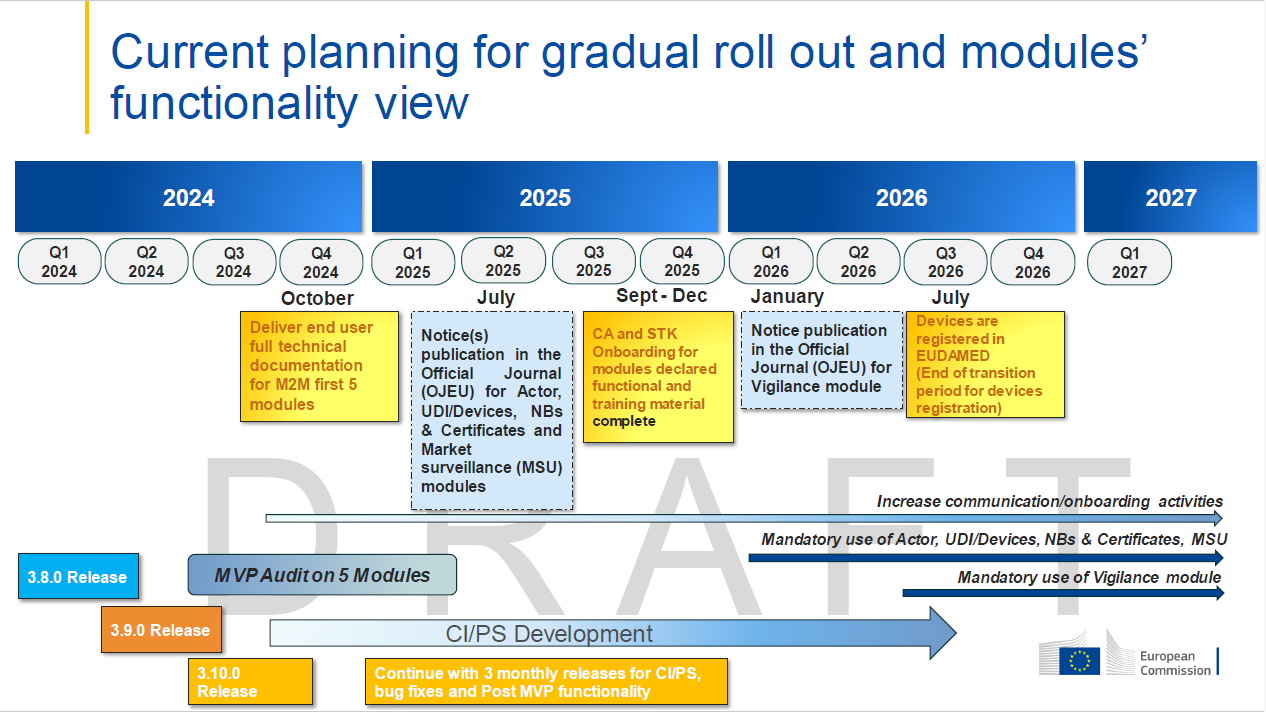
EU commission issues an updated planning for gradual roll out of the EUDAMED modules, however, the timeline for delivery of the clinical investigations / performance studies module has been set yet. In other words we are still stuck with local approaches when it comes to adverse event reporting to the regulatory bodies involved in clinical trials.